Rupa, the microbiologist, scanned the automated blood culture machine in her lab for positive vials. One of the vial slots flashed the red signal. Rupa quickly grabbed that vial and proceeded to run a gram stain test on the sample. Within the next two hours, she rang up the clinician to inform him that the growth was gram negative. “A stitch in time,” she told us, “The clinician can now cut down on the gram-positive coverage.” While her words reverberated the importance of narrowing the prescription down, her action underscored the role of a well-coordinated team effort in combating Antimicrobial Resistance (AMR)*.
Prescribing an antimicrobial isn’t a solo act. It’s a coordinated effort involving clinicians, microbiologists, infection control teams, and nurses. Together, they ensure that each prescription is appropriate, well-considered, and tailored to the patient’s needs. This collaborative process lies at the heart of Antimicrobial Stewardship frameworks, which help hospitals combat the misuse and overuse of these critical drugs. It’s a vital strategy in the global fight against AMR. Additionally, AMS frameworks ensure clarity and accountability within teams, while fostering a targeted and unified approach to combat the rising threat of drug-resistant pathogens. Every step in the right direction contributes to a cumulative progressive impact on public health outcomes.1
A study by Max Institute of Healthcare Management at Indian School of Business highlights vast variation in AMS frameworks across Indian hospitals, underscoring the complexity of implementing standardized approaches. While the larger hospitals have a structured approach, the medium and small sized hospitals may have semi-functional or non-existent frameworks. This lack of standardization leads to fewer checks on antibiotic prescription and dispensing, increasing the risk of misuse. Unchecked and under-regulated antimicrobial use is further compounded by unclear role definitions, stemming from inefficient AMS frameworks.1
In addition to a strong AMS framework, prescriber behaviour plays a crucial role in regulating the use of antimicrobials.1 However, prescriber behaviour is not shaped in isolation; it is deeply influenced by the strength and structure of the AMS framework itself. A weak or underdeveloped AMS framework can undermine prescriber accountability and decision-making. Absence of mechanisms that support and guide the prescribers, such as prescription justification forms, choice and saliency nudges and co-accountability with other healthcare staff, increase the chances of unwarranted prescriptions. This can have a direct bearing on the dispensing, escalation and de-escalation of antimicrobials, ultimately hampering the regulation of antimicrobial therapy and allowing AMR to persist and fester.
A prescriber encounters several critical junctures for antibiotic decision-making during the treatment process. These moments are not only clinically significant but also central to the success of antimicrobial stewardship… Four widely accepted key moments include: the moment of diagnosis; initiating cultures and empirical treatment; narrowing to low-spectrum antibiotics and revising the mode of administration (less invasive, intravenous to oral) after more microbiological and clinical data is available; and finally, after a clear diagnosis is reached deciding on the shortest effective antibiotic therapy for the patient.2 These steps are more than routine; they are opportunities to optimise antibiotic use and reduce the risk of fostering drug-resistant pathogens. In a world facing the growing threat of antimicrobial resistance (AMR), which directly causes over a million deaths and contributes to 4.95 million more each year,3 supporting prescribers at each of these decision points is essential for meaningful progress.
In India, the situation is unique and more complex. With an already heavy burden of diseases, weak health systems and largely unregulated antimicrobial pathways, the country faces an elevated risk of the adverse effects of AMR. This calls for specialised solutions. A four-moments approach, contextualized to the distinct Indian milieu, can ensure better checks and more accountability within the system.1 The approach involves a collaborative outlook to AMS, highlighting how key stakeholders can facilitate accountability and regulate drug use by stewarding the prescription behaviour. The figure below illustrates the four moments, the key actions and the role of stakeholders within each frame:
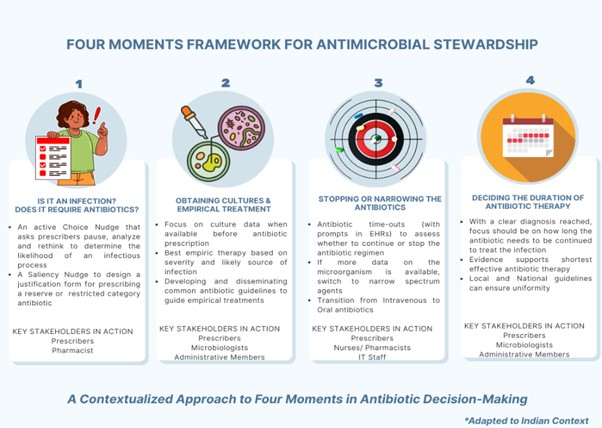
Nurses, pharmacists, microbiologists and lab technicians play as important a role as that of prescribers in preventing the misuse of antimicrobial drugs. They can act as gatekeepers and become a prescriber’s voice of reason at several critical moments in a treatment process, strengthening the AMS practices through pro-active teamwork. Additionally, administrative involvement in the form of outcome monitoring and quality audits is essential for the continuing evaluation of treatment practices. This process should serve not as punitive measure, but as a collaborative tool to refine the structure of AMS frameworks. Moreover, efforts like educating prescribers, improving communication, and integrating diverse stakeholders’ perspectives make AMS frameworks efficient and democratic. This supports the responsible use of antimicrobials and curtails the risk of AMR.1
Antimicrobial resistance (AMR) is not just a clinical challenge; it’s a reflection of underlying behavioural patterns within the healthcare teams. Prescribing behaviour, a key driver of unregulated drug use, is in turn a summation of diverse workflows and decision-making dynamics that vary from set-up to set-up in India’s diverse private healthcare landscape. This adapted four moments framework offers a pragmatic, flexible, and context-sensitive solution to recalibrate prescription practices. The framework isn’t about adding another layer of complexity. It’s about creating space for clarity. It respects existing workflows while nudging stakeholders toward more thoughtful, coordinated antimicrobial use.
But frameworks alone don’t drive change, people do. It’s time for every healthcare professional, including doctors, nurses, pharmacists, microbiologists, and administrators, to pause, reflect and then act. By embedding these four critical moments into daily decision-making, we can collectively reduce unnecessary prescriptions, harmonize practices across varied setups, and protect the efficacy of antimicrobials for future generations. Every prescription is a crucial decision, and every pause is a chance for critical reflection. The fight against AMR doesn’t need more drugs. It needs more thoughtful pauses.
*P.S. This narration is adapted from a quotation obtained during our study involving healthcare staff. To protect the privacy of individuals, names mentioned in this article may have been changed by the authors.
References:
- Max Institute of Healthcare Management, Indian School of Business. (2024). Antimicrobial Stewardship Programs in Health Facilities: A behavioural Science Approach. https://shorturl.at/XQ7q9
- Agency for Healthcare Research and Quality. (2019). Four Moments of Antibiotic Decision Making. https://www.ahrq.gov/antibiotic-use/acute-care/four-moments/index.html
- World Health Organization. (2023, November 21). Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance

Authors’ Bios’:
Samayita Ghosh
Senior Research Associate
Samayita Ghosh is a Senior Research Associate at Ashoka University and an Adjunct Faculty member at the Centre for Environmental Health, Public Health Foundation of India. With a strong foundation in public health research, she is also associated with ISB’s Max Institute of Healthcare Management (MIHM) as a Research Consultant. At MIHM, her work includes the project “Antimicrobial Stewardship Programs in Health Facilities: A Behavioural Science Approach” and an ongoing study on “Implementation Research on the Efficacy of Pill-in-Hand Adherence Monitoring for Tuberculosis.”

Deepali Verma
Research Analyst
Deepali Verma holds a doctorate in Anthropology and serves as a Research Analyst at ISB’s Max Institute of Healthcare Management. At MIHM, her work spans key public health research initiatives, including “Antimicrobial Stewardship Programs in Health Facilities: A Behavioural Science Approach” and the ongoing “Implementation Research on the Efficacy of Pill-in-Hand Adherence Monitoring for Tuberculosis.”

Navsangeet Saini
Writer
Navsangeet Saini is a communication professional with over 13 years of experience across academia, media and communication research, and writing. At the Max Institute of Healthcare Management, Indian School of Business (MIHM-ISB), she crafts narratives that make complex research accessible and relevant to diverse audiences.